 a)
a) b)
b)
INTRODUCTION
Soft x-rays, having a greater ability to penetrate biological material than electrons, have the potential for producing images of intact, living cells. In addition, by using the so-called 'water window' area of the soft x-ray spectrum, a degree of natural contrast is introduced into the image due to differential absorption of the wavelengths by compounds with a high carbon content compared to those with a greater oxygen content. The variation in carbon concentration throughout a cell therefore generates an image which is dependent upon the carbon density within the specimen. Using soft x-ray contact microscopy we have previously examined the green alga Chlamydomonas reinhardtii, and the most prominent feature of the cells are the numerous x-ray absorbing spheres, But they were not seen by conventional transmission electron microscopy [1,2]. Similar structures have also been reported by the Göttingen group using their cryo transmission x-ray microscope at BESSY [3]. Despite the fact that these spheres appear to occupy up to 20% or more of the cell volume when seen by x-ray microscopy, they are not visible by transmission electron microscopy. Given the difficulties and criticisms associated with soft x-ray contact microscopy, the present study was aimed at confirming the existence of these cellular inclusions and learning more of their possible chemical composition.
METHODS
For imaging, 2 µl of the spore suspension was placed between two120nm thick, Si3N4 windows. After selecting fields of view using light microscopy, the field coordinates were recorded and the cells imaged with soft x-rays using XM-1 on beamline 6.1 at the ALS, Berkeley, USA [4] using monochromatic soft x-rays (2.4 nm). For investigations of the oxygen content, images of air-dried material were taken on and below the oxygen absorption resonance energy (2.325 and 2.300 nm equivalent to 533 and 539 eV).
RESULTS & DISCUSSION
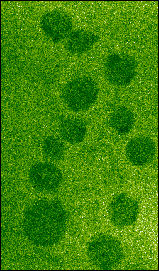
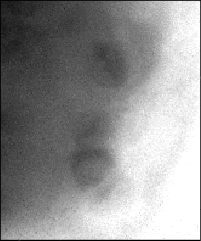
Soft x-ray transmission images of hydrated Chlamydomonas cells show, as with soft x-ray contact microscopy, that each cell contains several x-ray dense spherical inclusions (Fig. 1a). Because these spheres are denser than the surrounding cytoplasm, it is unlikely that they could be vacuoles since the carbon content would be expected to be less in the vacuole than in the surrounding cytoplasm. If these spheres are imaged a second time, clear evidence of their radiation sensitivity can be seen (Fig. 1b) as they appear to collapse leaving an x-ray dense deposit. This may be the membraneous material which previously surrounded these inclusions but which, when exposed to soft x-rays, is damaged such that the contents are lost and the remaining membrane then collapses. The sensitivity to soft x-ray exposure is particularly evident when the spheres were extruded from the cells by the pressure of the two silicon nitride windows (Fig. 2a-b).
 a)
a) b)
b)
 a)
a) b)
b)Spheres could not be identified in cells fixed in glutaraldehyde (i.e. as used in preparing for electron microscopy) since the increased density of the cytoplasm, due to the uptake of the glutaraldehyde, reduced the contrast between it and the spheres. In cells in which the increase in density was minimal, the spheres could still not be seen.
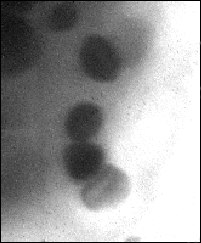
In dried material the spheres are visible when imaged at 539 eV and not at 533 eV photon energy, which indicates that oxygen is abundant in these spheres (Fig. 3). Since this material is dry the oxygen cannot be associated with the water content but other possibilities would include phosphates. There are reports of Chlamydomonas cells containing calcium polyphosphate granules [5], but the spheres seen in the present study appear to be too large. Furthermore, it is reported that the calcium is present in an insoluble form only in the gametes of Chlamydomonas, and in the vegetative cells (as used in the present study) calcium is diffuse within the cell [6]. When imaged at 2.4 nm, the amount of calcium that would be required to produce the relative x-ray absorbance of these spheres, compared to the cytoplasm, would be high. It would be expected that, if such levels of calcium existed, they would be detectable when imaging either side of the calcium edge (346 eV). However, no such absorption difference was detected (data not shown). Therefore our preliminary investigations suggest that calcium is not a major constituent of the spheres. Other possibilities could include some metal oxides since, under certain environmental conditions Chlamydomonas cells may accumulate metals such as manganese [7]. Once again, it is surprising that such large structures as these spheres are not seen by transmission electron microscopy.
 a)
a)
 b)
b)
Figure 3. Soft x-ray images of dried Chlamydomonas
cells taken below
(a: 533 eV=2.325 nm) and
at the oxygen absorption resonance
(b: 539 eV = 2.3 nm).
Image sizes: 10 µm x 10 µm. (61215051/52)
REFERENCES
This work has been supported by the U.S. Department of Energy, Office of Basic Energy Sciences, and the Office of Health and Environmental Research and the Laboratory Directed Research and Development Program of the E. O. Lawrence Berkeley National Laboratory.
Principal investigator: Tony Stead, Biological Sciences, Royal Holloway (University of London), Egham, Surrey, UK. TW20 0EX. E- mail: A.Stead@RHBNC.AC.UK. Telephone: (44) 1784 443761; Fax: (44) 1784 470756